Title
Daratumumab combined with Bortezomib, Cyclophosphamide and Dexamethasone for Treatment of Myeloma Patients Presenting with Extramedullary Disease. The Antares StudyStudy map
Overview / Summary
Study details
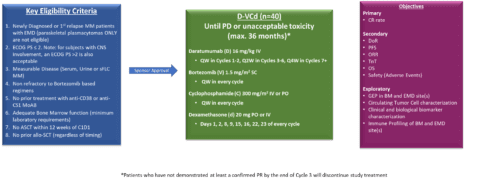
Patient eligibility criteria
- Newly Diagnosed or 1st relapse MM patients with EMN (paraskeletal plasmacytomas ONLY are not eligible)
- ECOG PS ≤ 2. Note: for subjects with CNS involvement, an ECOG PS > 2 is also acceptable
- Measurable Disease (Serum. urine or sFLC MM)
- Non refractory to bortezomib based regimens
- No prior treatment with anti-CD38 or anti-CS1 MoAB
- Adequate Bone Marrow function (minimum laboratory requirements)
- No ASCT within 12 weeks of C1D1
- No prior allo-SCT (regardless of timing)
Publications
No publications connected to this trial at the moment