Title
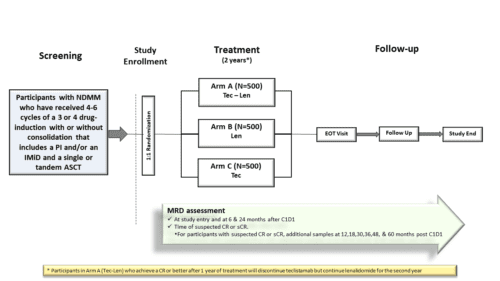
Phase 3 Study of Teclistamab in Combination With Lenalidomide and Teclistamab Alone versus Lenalidomide Alone in Participants With Newly Diagnosed Multiple Myeloma as Maintenance Therapy Following Autologous Stem Cell Transplantation – The Majestec-4 studyStudy map
Overview / Summary
The standard treatment for newly diagnosed fit adult patients is generally divided in different phases including induction treatment, autologous stem cell transplantation (ASCT), and maintenance treatment. In this study we want to evaluate how well the addition of Teclistamab to the standard maintenance treatment of Lenalidomide as well as Teclistamab alone work compared to the standard treatment of Lenalidomide alone. Approximately 1500 subjects will be randomized on a one to one to one (1:1:1) ratio to each of the following treatment group:
- Arm A Teclistamab added to the standard maintenance treatment with Lenalidomide
- Arm B standard treatment with Lenalidomide alone
- Arm C Teclistamab alone
Teclistamab subcutaneous inections will be given on a weight-based schedule. The treatment cycles have a duration of 28 days. The duration of the treatment will be approximately 2 years or earlier if progression or unacceptable toxicity occurs. Follow up visits will occur every 8 weeks for laboratory assessments. After the disease progression, follow up information will be collected every 4 months.
The primary objective of this study is to compare the efficacy of Teclistamab in combination with Lenalidomide (Tec-Len) with that of Lenalidomide monotherapy (Len), and the efficacy of Teclistamab monotherapy (Tec) with that of Lenalidomide monotherapy (Len) in the maintenance setting, as assessed by progression free survival (PFS).
Study details
Patient eligibility criteria
Approximately 1500 subjects (500 per arm ) will be randomized in a 1:1:1 ratio into 3 arms
The following criteria must be met before a subject will be enrolled in the study:
- ≥18 years of age (and the legal age of consent in the jurisdiction in which the study is taking place) at the time of informed consent
- Must sign an informed consent form (ICF) (in accordance with the local requirements) indicating that the participant understands the purpose of, and procedures required for, the study and is willing to participate in the study.
- Must have a new diagnosis of symptomatic multiple myeloma according to International Myeloma Working Group (IMWG) criteria (Appendix 4) and have received 4 to 6 cycles of 3 or 4 drug-induction therapy that includes a proteasome inhibitor and/or an IMiD with or without anti-CD38 monoclonal antibody and a single or tandem ASCT. Post‑ASCT consolidation is permitted for up to 2 cycles as long as the total number of induction plus consolidation cycles does not exceed 6.
- NOTE: Participants who receive up to the first 2 cycles of a 2-drug induction therapy may be eligible, provided the participants were deemed unable to tolerate a 3 or 4 drug therapy at start of treatment and the change to a 3 or 4 drug therapy was planned.
- Must have measurable disease at the time of diagnosis defined as measurable M‑protein in the serum (≥0.5 g/dL) or urine (≥200 mg/24h) or serum free light chain assay (defined as ≥10 mg/dL [≥100 mg/L] on involved light chain).
- Must have received only one line of therapy and achieved at least a partial response (≥PR) as per IMWG 2016 response criteria based on the investigator’s assessment. Participants with plasmacytomas at the time of diagnosis must meet IMWG 2016 response criteria for ≥PR based on repeat imaging utilizing the same modality (Kumar 2016).
- Must not have received any maintenance therapy.
- Must not be intolerant to the starting dose of Lenalidomide.